On 12 March 2020, the WHO declared the outbreak of the 2019 novel coronavirus, as a global pandemic. The WHO suggested the official name for the disease as corona-virus disease 2019 (COVID 19). The Coro-naviridae Study Group of the International Committee on Taxonomy of Viruses pro-posed the name of the virus as „severe acute respiratory syndrome corona-virus 2 (SARS‐CoV‐ II)‟
COVID‐ 19 is the third-known zoonotic disease from coronavirus, after severe acute respiratory syndrome (SARS) and Middle East respiratory syn-drome (MERS). Severe injury to the lung tissue can result in acute respiratory distress syndrome (ARDS) in patients with COVID infection, which can further precipitate septic shock. ARDS develops in 42% of patients presenting with COVID-19 pneumonia, and 61-81% of those requiring ICU care. The respiratory rate and SpO2 are two important parameters for judging patients‘ clinical condition, and allowing early recognition of ARDS. A patient who fits any one of the following conditions may have severe disease and requires fur-ther evaluation:
- Respiratory rate ≥ 30 breaths/min
- SpO2 ≤ 92 %
- PaO2/FiO2 ≤ 300 mmHg
Clinical manifestations can be viewed as a combination of the 2 processes, namely viral pneumonia and ARDS. COVID-19 ARDS is diagnosed when someone with confirmed COVID-19 infection meets the Berlin 2012 ARDS diagnostic criteria of:
- acute hypoxemic respiratory failure
- presentation within 1 week of worsen-ing respiratory symptoms;
- bilateral airspace disease on chest x-ray, computed tomography, or ultra-sound that is not fully explained by effusions, lobar or lung collapse, or nodules; and
- cardiac failure is not the primary cause of acute hypoxemic respiratory failure.
ARDS causes diffuse alveolar damage in the lung. There is hyaline membrane for-mation in the alveoli in the acute stage, and this is followed by interstitial widen-ing and edema and then fibroblast prolif-eration in the organizing stage.
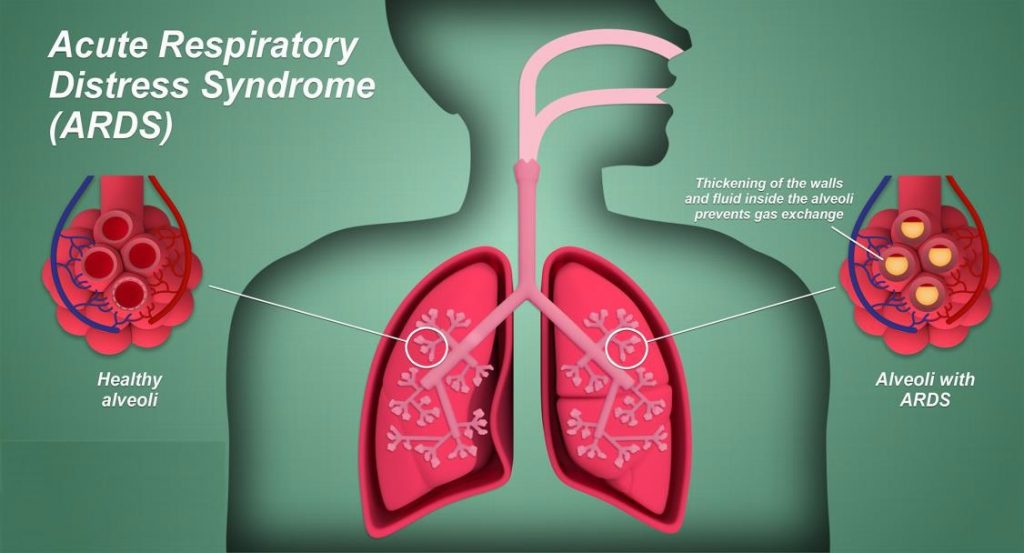
COVID-19-ARDS causes the typical ARDS pathological changes of diffuse alveolar damage in the lung. Pulmonary thrombosis is common in sepsis-induced ARDS. Coagulation dysfunction appears to be common in COVID-19, and is de-tected by elevated D-dimer. In fatal cases there is diffuse microvascular throm-bosis, suggesting a thrombotic microan-giopathy, and most deaths from COVID-19 ARDS have evidence of a thrombotic DIC.
COVID-19 ARDS appears to have worse outcomes than ARDS from other causes.
The ICU and hospital mortality from typical ARDS are 35.3% (95% CI, 33.3%-37.2%) and 40.0% (95% CI, 38.1%-42.1%), respectively. For COVID-19 ARDS mortality ranged between 26% to 61.5% if ever admitted into a critical care setting, and in patients who received mechanical ventilation, the mortality can range between 65.7% to 94%.
Risk factors for poor outcomes include older age, presence of comorbidities such as hypertension, cardiovascular disease, and diabetes mellitus, lower lymphocyte counts, kidney injury and raised D-dimer. Death from COVID-19 ARDS is due to respiratory failure(53%), respira-tory failure combined with cardiac fail-ure(33%), myocardial damage and circulatory failure(7%), or death from an unknown cause.
Management
COVID-19 ARDS is a predictable serious complication of COVID-19 that requires
early recognition and comprehensive management. The strategy of breathing support is very important in treating COVID-19 ARDS. The key elements are:
- Use oxygen by nasal cannulae to achieve SpO2>92%. Before endotracheal intubation, it is im-portant to consider a trial of high-flow nasal oxygen for patients with moderately severe hypoxaemia. This procedure might avoid the need for intubation and mechanical ventila-tion because it provides high con-centrations of humidified oxygen, low levels of positive end expiratory-pressure, and can facilitate the elim-ination of carbon dioxide.
- For patients with COVID-19 who require endotracheal intubation, use of low tidal volume (6 mL/kg per predicted bodyweight) with a plat-eau airway pressure of less than 30 cm H2O, and increasing the respira-tory rate to 35 breaths per min as needed, is the mainstay of lung pro-tective ventilation
- Prone ventilation works- Placing a person in prone position promotes more homogenous aeration of the lung in ARDS and can improve oxy-genation. suggested use is for > 12 hours per day.
- Consider ECMO for rescue- Veno-venous extracorporeal membraneox-ygenation (vvECMO) can be used as rescue for mechanically ventilated adults with COVID-19 and hypoxae-mia that persists despite optimized ventilation, use of rescue therapies and prone ventilation. The WHO recommends usage of extracorporeal membrane oxygenation (ECMO) in patients that sustain hypoxia refrac-tory to supplementary oxygen
- Avoid Non-invasive Ventilation- The lung protective ventilation strategy used in typical ARDS involves a low tidal volume (6mls/kg) and higher PEEP targets. For COVID-19 ARDS, a change to more generous tidal volume targets allowing up to 8mls/kg, and lower PEEP levels is suggest-ed to prevent Patient-Self inflicted lung injury (P-SILI).
- Adjunct treatment -In the absence of shock, fluid conservative therapy is recommended to achieve a negative fluid balance of 0·5 to 1·0 L per day. In the presence of shock, fluid bal-ance might be achieved with renal replacement therapy, especially if there is associated acute kidney inju-ry and oliguria. Antibiotics should be considered since secondary bac-terial infections have been reported in patients with COVID-19.
In COVID-19 ARDS, the evidence for systemic steroids is still scarce and it is only recommended in patients with con-comitant shock which has been unre-sponsive to vasopressors. There are con-cerns that steroids may increase viral shedding and possibly lead to a higher mortality rate.
Alternatively, convalescent plasma and IgG are used as rescue therapy in critical Cases.


